Pipeline & Indications
Expanding Therapeutic Horizons for Hard-to-Treat Diseases
We are dedicated to tackling unmet medical needs in antimicrobial resistance and oncology
by developing lipid-based therapeutic

Pipeline & Indications
Expanding Therapeutic Horizons for Hard-to-Treat Diseases
We are dedicated to tackling unmet medical needs in antimicrobial resistance and oncology by developing lipid-based therapeutic

Anti-Microbial Resistance:
A Silent Pandemic
Addressing the urgent need for next-gen antimicrobial solutions
Antimicrobial resistance (AMR), a “silent pandemic,” is one of the top global health threats, estimated to be directly responsible for 1.45 million deaths worldwide and projected to cause 40 million deaths by 2050.
1.45 M
deaths worldwide
40 M
deaths by 2050
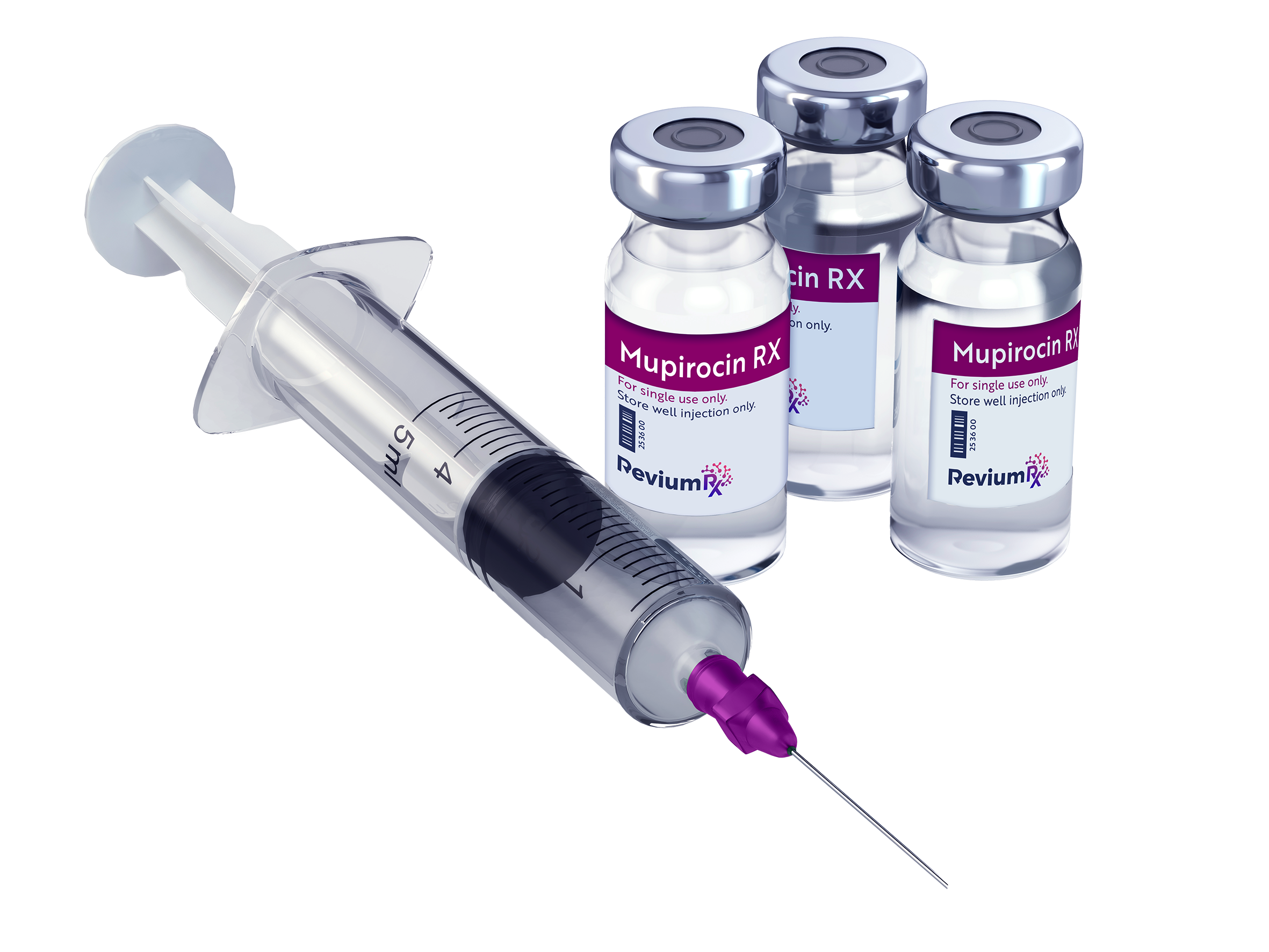
The Solution:
Revium Rx Nano-Mupirocin
Revium Rx’s nano-mupirocin is a liposomal formulation of the approved, highly effective antibacterial drug mupirocin, which is currently limited to topical use. If approved, the novel proprietary formulation of nano-mupirocin will be administered systemically, providing extended presence in the bloodstream and enhanced delivery of mupirocin to inflamed areas of the body.
Promising Pre-Clinical Results
Preclinical studies by the National Institutes of Health demonstrated that nano-mupirocin has low minimum inhibitory concentration (MIC) levels and effectiveness against antibiotic-resistant strains, including MRSA and VRE.

Immunization
The critical need for rapid vaccine development
in the case of a pandemic
The COVID-19 pandemic highlighted the critical need for rapid vaccine development, demonstrating that global collaboration can save millions of lives. Yet for many emerging infectious diseases, effective vaccines remain limited, and traditional development faces biological and commercial challenges.
The Solution: Revium’s Lipid-Based Protein-Loaded Technology (LPLT)
Our adaptable LPLT platform uses proprietary lipid assemblies to enhance immune responses in preclinical infectious disease models. By modulating the immune system, LPLT has the potential to improve vaccine efficacy, durability, and safety. Building on lessons from COVID-19, Revium aims to accelerate next-generation vaccines for high-priority threats such as West Nile virus and Zika virus.
Results
Early studies indicate that LPLT could offer a scalable, adaptable strategy to fill critical gaps in vaccine innovation and protect vulnerable populations worldwide.